This is the final of a three-part post on our work on the anoles of Cay Sal Bank, Bahamas. In this post, I will visit the Brown Anoles (Anolis sagrei). Like many, many places in the Caribbean, Anolis sagrei occurs across the Cay Sal Bank. This species has the widest range of any Caribbean anole, having colonized a huge range of regions from ancestral origins in Cuba- from the northern Bahamas, throughout the northern Caribbean, all the way to the Atlantic versant of Mesoamerica.

(Mostly) native range of Anolis sagrei.
Our ongoing work on this species has resolved the evolutionary history of A. sagrei across this great range, but one hole that had lingered was the status of the populations on the Cay Sal Bank. Prior to our cruise to the region in 2015, A. sagrei was known from the following islands: Cay Sal Island, the Anguilla Cays (including Cotton Cay), and Elbow Cay (Buckner et al. 2012). Further, these populations were considered to be the subspecies A. sagrei ordinatus, or, the Bahamian Brown Anole (Buden and Schwartz 1968; Buckner et al. 2012). This subspecies was originally described owing to having supraorbital scales in contact and a different dewlap color. We know now that dewlaps are highly variable both among and within populations of brown anoles on the Bahamas banks (e.g., Vanhooydonck et l. 2008). Populations proximal to the Cay Sal Bank- that is- populations on the Bimini islands, have a very distinct dewlap comprised of a light orange background streaked with dark red. Brown anoles on Cay Sal do not share this dewlap color; instead, they have a more classic sagrei pattern of darker red with a light distal border. This is not a smoking gun for considering Cay Sal anoles something other than A. s. ordinatus, of course, given the range of dewlaps we see to the east.

Cay Sal (left), South Bimini (right). Photos by R. Graham Reynolds.
If Cay Sal browns were indeed A. s. ordinatus, that would imply a (likely) westward colonization across the Santaren Channel–not an implausible scenario. During periods of lower sea level, the Cay Sal Bank would have been a big ‘ol target for lizards involuntarily leaving the Great Bahamas Bank. Of course, an alternative would be the reverse: an initial colonization of Cay Sal, followed by dispersal to the east across the Channel. Of relevance, during the course of the work I’m presently describing, we also found a snake: Tropidophis. We determined, using the same molecular phylogenetic techniques, that this snake is most likely T. curtus, and thus a population conspecific with Tropidophis over on the Great Bahamas Bank, evidence for a likely westward colonization.
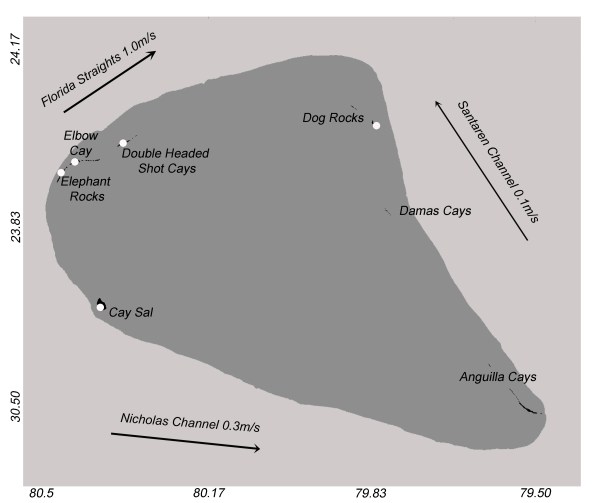
Map of the Cay Sal Bank, from Reynolds et al. (2018). Note that Cotton Cay is part of the Anguilla Cays.
Of course, Cay Sal browns could also be Cuban in the sense that they might have colonized the bank directly from Cuba across the Nicholas Channel. To parse these alternative origin stories, we collected samples of the species from across the Cay Sal Bank and generated a coalescent gene tree paired with all our sampling from other brown anole populations across the region. We find that Cay Sal A. sagrei are actually much more closely related to western Cuba A. sagrei, rather than Bahamas A. s. ordinatus. Combining this finding with our analysis of A. fairchildi, we find that this particular Anole Outpost was colonized from Western Cuba by at least two species–and likely at different times.

Phylogeny of A. sagrei, showing Cay Sal Bank lineagers in blue (and a Cay Sal specimen in the inset). From Reynolds et al. 2018.
New Records
In addition to these findings, we also documented some novel populations of A. sagrei on the Cay Sal Bank. We added East Doubled Headed Shot Cay, Elephant Rocks, Great Dog Rock to the list of known populations on the bank. What is particularly interesting about these new records is the range of habitat types that they support. Cay Sal Island and the Anguilla Cays are by far the most lush, with lots of vegetation. To the north, the cays become increasingly xeric and barren. East Double Headed Shot Cay is the most vegetated of the northern islands, and has a thick, but low, covering of coastal shrub plant community. Anolis sagrei is not abundant on this island, and we only saw a few dozen during several hours of searching.

East Doubled Headed Shot Cay. Photo by R. Graham Reynolds.
In stark contrast, the Elephant Rocks to the west are tall, jagged, steep, and rocky islets with almost no vegetation at all. We had low expectations as we jumped into the sea from the dingy to start our ascent of these islands at dawn. But, to our surprise, we found some anoles happily living among the rocks. Not at high densities, but here they were, a saxicolous population of A. sagrei.

Elephant Rocks, Cay Sal Bank. Photo by R. Graham Reynolds.
Naturally, Alberto and I would love to follow up on some of this, but Cay Sal is a tough place to work. Maybe someday we’ll get back there, in the meantime, we can reflect on what a special opportunity we had to visit this Anole Outpost.

Sunrise on the Cay Sal Bank. Photo by R. Graham Reynolds.
References
Buckner, S. D., R. Franz, and R. G. Reynolds. 2011. Bahama Islands and Turks & Caicos Islands. In R. Powell and R. W. Henderson, editors. Island Lists of West Indian Amphibians and Reptiles. Bulletin of the Florida Museum of Natural History 51: 85–166.
Buden, D. W., and A. Schwartz. 1968. Reptiles and birds of the Cay Sal Bank, Bahama Islands. Quarterly Journal of the Florida Academy of Sciences 31: 290–320.
Vanhooydonck, B., A. Herrel, J. J. Meyers, and D. J. Irschick. 2009. What determines dewlap diversity in Anolis lizards? An among-island comparison. Journal of Evolutionary Biology 22: 293–305.





















